how many valence electrons do halogens have|Determine valence electrons using the periodic table : Pilipinas Valences of the Elements Chemistry Table. You may assume that the . Phil Jackson (born September 17, 1945, Deer Lodge, Montana, U.S.) is an American professional basketball player, coach, and executive. . a member of the Knicks through the 1977–78 season and then finished out his career with two seasons as a New Jersey Net. Britannica Quiz. Great Moments in Sports Quiz.
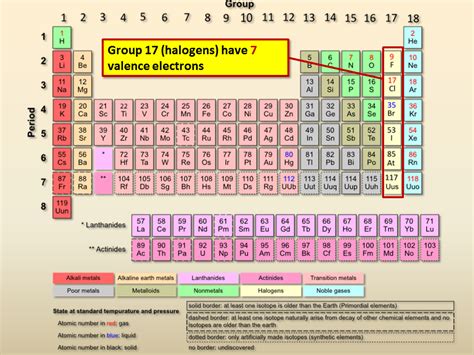
how many valence electrons do halogens have,Because the halogen elements have seven valence electrons, they only require one additional electron to form a full octet. This characteristic makes them more reactive than other non-metal groups. Introduction. Halogens form diatomic molecules (of .The halogens all have the general electron configuration \ (ns^2np^5\), giving them .

Halogens are the most reactive non-metals on the periodic table, with 7 valence electrons in their outermost shell. Learn more about the properties, reactivity, and locations of the halogens in group 17 of the . The halogens all have the general electron configuration \ (ns^2np^5\), giving them seven valence electrons. They are one electron short of having the full outer \ (s\) and \ (p\) sublevel, which makes them . Valences of the Elements Chemistry Table. You may assume that the . Because the halogen elements have seven valence electrons, they only .
Now, the halogens have seven valence electrons. So you can imagine, they're only one electron away from having an electron configuration like the noble gas to the right of .
Each Halogen ends in s2p5 with 7 valence electrons. I hope this was helpful. SMARTERTEACHER. Answer link. The Halogens (F, Cl, Br, I, At) are found in column 17 .
Key Questions. How do valence electrons determine chemical properties? Answer: It is not the valence electrons themselves, but the number of valence electrons that .
Tell which group of elements (alkali metals, alkaline earth metals, halogens, noble gases) has atoms with the specified number of valence electrons? 1 valence . sulfur. helium. potassium. aluminum. Solution. Sulfur (S) is located in Group VIA (Group 16), so it has 6 valence electrons. Helium (He) is located in Group VIIIA (Group 18). However, one atom only has two electrons, so it could never have more than 2 valence electrons. As noted above, helium is the only exception for the main group . Q: Based on their position in the periodic table from the Figure above, how many valence electrons do you think halogens have? A: The number of valence electrons starts at one for elements in group 1. It .
In the periodic table the halogens make up Group 17 (according to the numbering system adopted by the International Union of Pure and Applied Chemistry), the group immediately preceding the noble gases. The .And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system for classifying groups. So one, two, three, four, five, six, seven, and eight. So we're going to ignore the other way to number the groups.
If you want a Periodic table with Valence electrons, then visit Periodic table with Valence electrons labeled in it. (Where you will get the HD images along with the explanation). Valence Electrons Chart for All Elements. Atomic number Elements Valence electrons; 1: Hydrogen (H) 1: 2: Helium (He) 2: 3: Lithium (Li) 1: 4: Beryllium .The Halogens. There are six elements in Group VIIA, the next-to-last column of the periodic table. As expected, these elements have certain properties in common. . and iodine have valence shell d orbitals and can expand their valence shells to hold as many as 14 valence electrons. 4. The chemistry of the halogens is dominated by oxidation .Determine valence electrons using the periodic table Position of Halogens in the Periodic Table. The halogens constitute group VIIA of the periodic table. Each halogen has seven valence electrons. The outermost electronic configuration of halogen is represented by the general formula ns 2 np5. All elements, with the last electron being added to one of the orbitals of their p-subshell, therefore find their .Overview Electron configuration. The electrons that determine valence – how an atom reacts chemically – are those with the highest energy.. For a main-group element, the valence electrons are defined as those electrons residing in the electronic shell of highest principal quantum number n. Thus, the number of valence electrons that it may have .

The halogens all have the general electron configuration \(ns^2np^5\), giving them seven valence electrons. They are one electron short of having the full outer \(s\) and \(p\) sublevel, which makes them very reactive. . The halogens all have seven electrons in their outer shells. The electron configuration in the outer shell is \(ns^2np^5\).how many valence electrons do halogens have Figure 15.4.3 15.4. 3: The ammonium ion. When drawing the Lewis structure of a polyatomic ion, the charge of the ion is reflected in the number of total valence electrons in the structure. In the case of the ammonium ion: 1 N 1 N atom = 5 = 5 valence electrons. 4H 4 H atoms = 4 × 1 = 4 = 4 × 1 = 4 valence electrons.Flexi Says: The halogens also form single covalent bonds in their diatomic molecules. An atom of any halogen, such as fluorine, has seven valence electrons. Its unpaired electron is located in the 2 p orbital. Discuss further with Flexi. Ask your own question! The halogens also form single covalent bonds in their diatomic molecules. An . The halogens all have the general electron configuration ns 2 np 5 , giving them seven valence electrons. Do all halogens have valence electrons? The halogens are among the most reactive of all elements, although reactivity declines from the top to the bottom of the halogen group. Because all halogens have seven valence electrons, .Answer and Explanation: 1. Become a Study.com member to unlock this answer! Create your account. View this answer. Halogens have seven electrons in their outer shells. Electrons are the negatively charged particles that orbit the nucleus of an atom in various. See full answer below. One way to categorize the elements of the periodic table is shown in Figure 3.8.1 3.8. 1. The first two columns on the left and the last six columns on the right as mentioned earlier are the main group elements. The ten-column block between these columns contains the transition metals.
Valences of the Elements Chemistry Table. You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.Halogens have 7 valence electrons. Halogens are the group (vertical column) of elements on the Periodic Table that lie 2nd from the from right side. They sit next to the noble gases, which have 8 .All alkaline earth metals react vigorously with the halogens (group 17) to form the corresponding halides (MX 2). . As expected for compounds with only four valence electrons around the central atom, the beryllium halides are potent Lewis acids. They react readily with Lewis bases, such as ethers, to form tetrahedral adducts in which the . Study with Quizlet and memorize flashcards containing terms like How many valence electrons do all halogens have?, Element A is located between group 16 and noble gas on the periodic table. A is a(n), Which other elements of the periodic table do halogens readily react with to form halide salts? and more.
how many valence electrons do halogens have|Determine valence electrons using the periodic table
PH0 · What is the valence electron configuration for halogens?
PH1 · Valences of the Elements Chemistry Table
PH2 · Valence electrons and ionic compounds (video)
PH3 · Valence Electrons
PH4 · Group 17: The Halogens
PH5 · Group 17: The Halogens
PH6 · Group 17: General Properties of Halogens
PH7 · Determine valence electrons using the periodic table
PH8 · 6.12: Halogens
PH9 · 10.6: Valence Electrons